
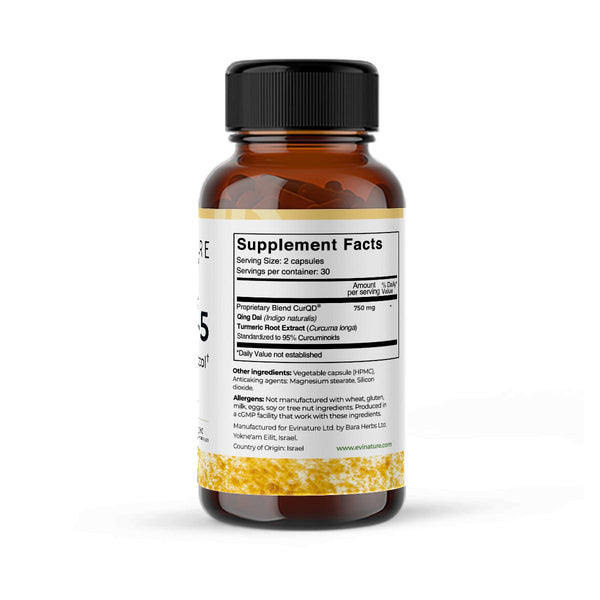






CurQD-1
CurQD-1
CurQD1 contains a carefully balanced blend of herbal extracts that has been studied for its role in supporting general gut comfort and overall digestive wellbeing.
Money Back Guarantee
Free Shipping i
Free shipping to the United States, Europe, Canada and South AmericaPatent Pending
Not tested on animals
Lab Tested by third party
Crafted from naturally derived ingredients
Couldn't load pickup availability
Developed by Leading Researchers
The CurQD® Protocol was developed by Nir Salomon, Director of the Integrative Gastroenterology Unit at Sheba Medical Center (SMC), and Professor Shomron Ben-Horin MD, Chief of the Gastroenterology Department & Director of the Gastro-Immunology Research Laboratory at SMC.
The protocol is based on years of clinical experience and research conducted at Sheba Medical Center, recognized by Newsweek’s as one of the top 10 hospitals worldwide.
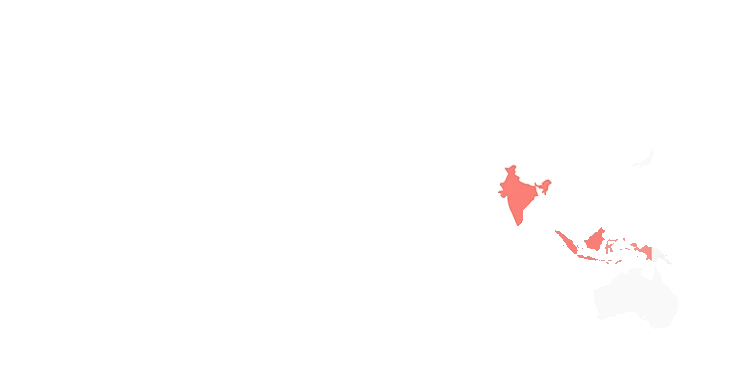
Origin & Harvest calendar
Curcumin is grown in India, and harvested in the winter, “when the sprouts have withered”.
Qing Dai is native to and grown in Southern Russia, China, Eastern Europe, and Northern America. It is harvested July and August.
Jan
Feb
Mar
Apr
May
Jun
Jul
Aug
Sep
Oct
Nov
Dec
Warnings
Do not use if you have any liver disease, including but not limited to, autoimmune hepatitis or primary sclerosing cholangitis (PSC).
Allergen Warning:
May contain cereals with gluten, celery, soybeans, nuts, mustard, sesame seeds, molluscs.
Does not contain:
Eggs, fish, peanut, milk, lupin, crustations, sulfur dioxide & sulphites at a concentration of more than 10 mg/kg or 10 mg/1 expressed as S02.
Disclaimer:
Consult your physician before use if you are pregnant, nursing, have a medical condition or are taking any medications. Should only be used as directed. Keep out of reach of children. Store in a cool and dry place.
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
FAQ
Are there any side effects?
* Headaches - commonly experienced in the first 72 hours of treatment.
*Mild transient elevation of liver enzymes - blood tests are recommended 3-6 weeks after starting CurQD (assessing ALT and AST).
* Shortness of breath.
* Chest pain.
*Palpitations (a sense of strong, irregular heartbeat).
* Jaundice.
* Any unexplained symptom.
* Qing Dai has on rare occasions been associated with pulmonary arterial hypertension (PAH) which dissipated upon discontinued use. Zero cases have been reported with CurQD.
Please note this is given only as general information and we always recommend consulting with your physician regarding any treatment decisions.
Can I take CurQD® with my current treatment or medication?
Is CurQD® safe for children?
Can I take CurQD® when pregnant?
Sign Up
Stay informed with the latest trials, Studies and fresh arrivals:
- Choosing a selection results in a full page refresh.
- Opens in a new window.


